Scientists identify a hormone that modulates innate behavior in flies
Published in Nature Communications, the study was made in collaboration with researchers of CONICET and CEDOC.
The research team comprises scientists from the Instituto de Investigaciones Bioquímicas de Bahía Blanca (INIBIBB, CONICET-UNS) and the Centro de Estudos de Doenças Crónicas de Lisboa (CEDOC). The study reveals that Dilp8, a hormone of the insuline family, has a key role in the proper execution of innate complex behaviours, those that like the sucking of babies at birth, do not require neither learning nor previous experience to perform.
Although it was known that this type of behavior consists of a succession of genetically connected motor and physiological processes, how these mechanisms are coupled and organized was not revealed. In order to understand this, a team led by Andrés Garelli, CONICET researcher, focused on the study of puparation, an innate behavior of fly larvae that develops in several steps and is vital for their survival during metamorphosis.
The flies used in the experimental stage of the study are known as fruit flies, but their scientific name is Drosophila melanogaster. It is a species that has been used for more than a hundred years in research areas because they are easy to grow in the laboratory and have a short life cycle: the larvae become reproductive adults in only ten days. Besides, they can be used to examine some of the basic processes of biology, such as cell division, aging, genetic information processing, which are similar in all organisms.
“It is estimated that about 70 percent of human genes that are related to diseases have a similar gene -homologous- in Drosophila, which participates in the same process. Thanks to this homology, and the versatility of the current genetic tools, Drosophila melanogaster is an appropriate experimental model to analyze human pathologies and specific processes in invertebrate animals at the molecular level, as in this case,” explains Yanel Volonté, CONICET postdoctoral fellow at the NIBIBB, and one of the authors of the study published in Nature Communications.
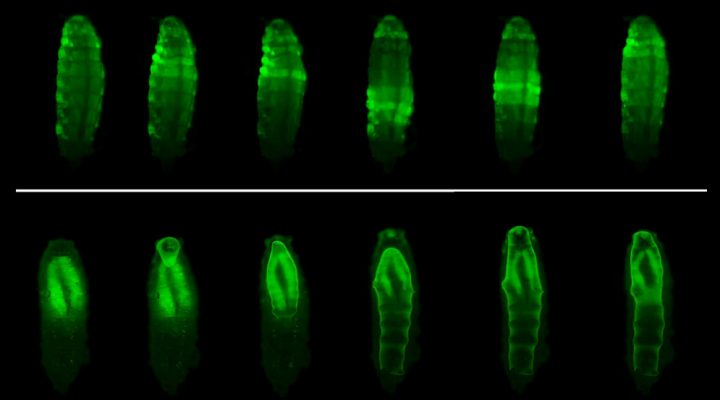
The study began from a serendipitous finding. “We were using these flies to understand how certain genes coordinate in the growth of different parts of the body. So we analyzed mutations. In this case we had removed the genes that encode a hormone (Dilp8) and neuronal receptor (Lgr3) and we noticed that the shape of the pupal envelope, which is the rigid cover that forms just before the start of the metamorphosis was different from the usual one,” Volonté describes.
Like other insects, Drosophila larvae completely change their shape during metamorphosis. In their larval stage, its appearance is similar to that of a worm, while in its adult stage, the fly has a head, thorax and abdomen with legs and wings.
The metamorphosis process occurs within a rigid cover, the puparium, which protects them from desiccation and predators, while allowing the exchange of oxygen with the environment. The puparium is formed by the transformation of the cuticle -the outermost layer- of the larva, which is at first flexible and hardens by chemical transformations. This takes place simultaneously with a series of morphological changes made by muscle contractions.
“We found that these two processes are coupled by the action of Dilp8, which makes this happen in a coordinated manner. This hormone is produced in the epidermis of the larva at the time the cuticle begins to harden. When this hormone reaches the brain, it triggers the complex movements pattern that changes the shape of the body. If Dilp8 is not present, the cuticle still hardens but retains the shape of the larva. Then, a defective covering is formed and that leads to the death of the animal,” Garelli explains.
“There are around 180 neurons, out of the 10,000 that make up the larval brain, that express the neuronal receptor Lgr3 and therefore could be activated in response to Dilp8. Nevertheless, only six are sensitive to the hormone. After having identified the molecular and cellular components that transmit the signal to the central nervous system, we can examine how this signal is processed and translated into the coordination of the activity of the motor centers,” says the researcher and concludes: “Our study opens new paths to answer new questions related to the neuromodulatory role of neuropeptides in behavior.”
By Pía Squarcia
References
Heredia, F., Volonté, Y., Pereirinha, J. et al. The steroid-hormone ecdysone coordinates parallel pupariation neuromotor and morphogenetic subprograms via epidermis-to-neuron Dilp8-Lgr3 signal induction. Nat Commun 12, 3328 (2021). https://doi.org/10.1038/s41467-021-23218-5